General properties of enzymes:
· Higher reaction rates
· Milder reaction conditions
· Greater reaction specificity
· Capacity for regulation
Mechanism of enzyme catalysis:
Enzymes achieve their enormous rate acceleration via the same catalytic mechanisms used by the chemical catalyst. However, enzymes have simply been better designed through evolution. Enzymes like other catalysts reduce the free energy of the transition state (delta G) i.e., they stabilize the transition state of the catalyzed reaction. What makes enzyme such effective catalyst is their specificity of substrate binding combined with their arrangement of the catalytic group. The match can be learned about the enzymatic reaction, mechanisms by examining the corresponding non-enzymatic reaction of model compounds. At all times, the rules of chemical reason apply to the system.
The types of catalytic mechanisms that enzymes employ have been identified as:
1. Acid-base catalysis
2. Covalent catalysis
3. Metal-ion catalysis
4. Electrostatic catalysis
5. Proximity and orientation effects
6. Preferential binding of the transition state complex.
ACID-BASE CATALYSIS:
General acid catalysis is a process in which partial proton transfer from an acid lowest the free energy of a reaction transition state. For e.g., a un catalyst keto-enol tautomerization reaction occurs slowly as a result of the high free energy of its carbanion like transition state.
Proton-donation to the oxygen atom reduces the carbanion character of the transition state thereby accelerating the reaction. A reaction can be stimulated by general base catalyst if its rate is increased by partial proton abstraction by the base. Some reactions may be simultaneously subject to both processes; these are concerted acid-base catalyst reactions.
NOTE: Many types of biochemical reactions are susceptible to acid/base catalysis. The side chains of the amino acid residues Asp, Glu, His, Lys have pKs in or near the physiological pH range, which permits them to act as an acid/base catalyst. The ability of enzymes to arrange several catalytic groups around their substrates makes concerted acid-base catalyst a common enzymatic mechanism. The catalytic activity of these enzymes is sensitive to pH since the pH influences the state of protonation of side chains at the active site.
Bovine pancreatic RNase A provides an example of enzymatically mediated acid-base catalysis. This digestive enzyme is secreted by the pancreas into the small intestine where it hydrolyzes RNA to its component nucleotides.
The isolation of 2’, 3’ –cyclic nucleotides from RNase-A digest of RNA indicates that 2’,3’- cyclic nucleotides are intermediates in the RNase A reaction. The pH dependence of the rate of RNase A reaction suggests the involvement of two ionizable residues. This information together with the chemical derivation and X-ray studies indicate that RNase A has two essential histidine residues, his12 and his119 that act in a concerted manner as a general acid-base catalyst.
The RNase A reaction is a two-step reaction:
· His 12 acting as a general base abstracts the proton from RNA 2’-OH group. Thereby, promoting its nucleophilic attack on the adjacent phosphorus atom. His 119 acting as a general acid promotes bond-scission by protonating the leaving group.
· The 2’,3’- cyclic intermediates are hydrolyzed through what is essentially the reverse of the first step in which water replaces the leaving group. Thus, his12 now acts as a general acid and his119 as a general base to yield the hydrolyzed RNA and the enzyme in its original state.
COVALENT CATALYSIS:
Covalent catalysis accelerates reaction rates through the transient formation of a catalysts-substrate covalent bond. Usually, this covalent bond is formed by the reaction of a nucleophilic group on the catalyst with an electrophilic group of the substrate and hence this form of catalysis is often called nucleophilic catalysis.
The decarboxylation of acetoacetate, chemically catalyzed by primary amines is an example of such a process.
Covalent catalysis can be conceptually decomposed into 3 stages;
1. The nucleophilic reaction between the catalyst and the substrate to form a covalent bond.
2. The withdrawal of electrons from the reaction center by the now electrophilic catalyst.
3. The elimination of the catalyst, a reaction that is essentially the reverse of step 1.
the mechanism of nucleophilic catalysis resembles that of base catalysis except that instead of abstracting an H+ from the substrates the catalyst nucleophilically attacks the substrate to form a covalent bond.
NOTE: Biologically important nucleophiles are negatively charged or they contain unshared electron pairs that easily form covalent bonds with electron deficient centers. Electrophiles, in contrast, include groups that are positively charged or contain an unfilled valence electron shell or contain an electronegative atom.
· An important aspect of covalent catalysis is that the more stable the covalent bond formed, the less easily it can decompose in the final steps of a reaction. Good covalent catalysis must, therefore, combine the seemingly contradictory properties of high nucleophilicity and the ability to form a good leaving group i.e., to easily reverse the bond formation step.
· Groups with high polarizability (high mobile electrons) such as imidazole and thiol function groups have these properties and hence make good covalent catalyst.
· Such functional groups in proteins include the imidazole group of histidine, the thiol group of cysteine, the carboxyl group of Asp, hydroxyl group of serine. In addition, several coenzymes thiamine pyrophosphate (TPP) and pyridoxal phosphate, function in association with their apoenzymes as a covalent catalyst.
METAL-ION CATALYSIS:
Nearly one-third of all known enzymes metal ions for catalytic activity. This group of enzymes includes the metalloenzymes which contain tightly bound metal ion cofactors. Most commonly transition metal ions such as Fe2+, Fe3+, Cu2+, Zn2+, Mn2+, Co2+. Metal activated enzymes in contrast loosely bind metal ions from solution, usually the alkali and alkaline earth metal ions like Na+, K+, Mg2+, Ca2+.
In this group of enzymes, the ions often play a structural rather than a catalytic role. Metal ions participate in the catalytic process in three major ways:
1. By binding to the substrate to orient them properly for reaction.
2. By mediating oxidation-reduction reactions through reversible changes in the metal ions oxidation state.
3. By electrostatically stabilizing or shielding negative charges.
In many metal ion catalyzed reactions the metal ion acts in much the same way as a proton to neutralize negative charges. Metal ions are often much more effective catalyst then protons metal ions can be present in a higher concentration at neutral pH and may have charges greater than +1.
A metal ions charge also makes it bound to H2O molecule more acidic than free H2O and therefore a source of nucleophilic OH- ions even below neutral pH. An example of this phenomenon occurs in the catalytic mechanism of carbonic anhydrase an enzyme that catalyzes the
ELECTROSTATIC CATALYSIS:
The binding of the substrate generally excludes H2O from an enzymes active site. The active site has a polarity characteristics of an organic solvent where electrostatic interactions are much stronger than they are in aqueous solution.
Although experimental evidence and theoretical analysis on the subject are still sparse, the charge distributions around the active site of enzymes seem to be arranged so as to stabilize the transition state of the catalyst. This mode of rate enhancement which resembles metal ion catalysis is termed electrostatic catalysis. Moreover, in several enzymes, charge distribution apparently guides polar substrate towards their binding site to further enhance the reaction rates.
CATALYSIS THROUGH PROXIMITY AND ORIENTATION EFFECT:
Although enzymes employ catalytic mechanisms that resemble those of organic model reactions, they are far more catalytically efficient than these models. Such efficiency must arise from the specific physical conditions at enzyme catalytic sites that promote the corresponding chemical reactions. The most obvious effects are proximity and orientation. Reactants must come together with a proper special relationship for a reaction to occur by simply binding their substrates enzyme to facilitate their catalyze reactions in three ways:
1. Enzymes bring substrates into contact with their catalytic group and in reactions with more than one substrate with each other.
2. Enzymes bind their substrate in the proper orientation for reaction. Molecules are not equally reactive in all directions. Rather they react most readily if they have proper relative orientation. It is estimated that properly orientating substrates can increase reaction rates by a factor of up to 100.
3. Enzymes freeze out the relative translational and rotational motions of their substrates and catalytic groups.
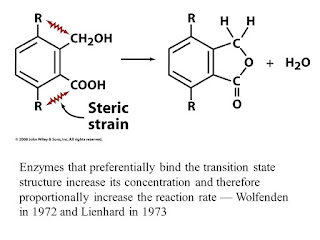
CATALYSIS BY PREFERENTIAL TRANSITION STATE BINDING:
An enzyme may bind to the transition state of the reaction it catalyzes with greater affinity than its substrates or products. the original concept of transition state binding proposed that enzymes mechanically strain their substrate towards the transition states geometry through binding sites into which undistorted substrates did not properly fit. Enzymes that preferentially bind the transition state structure to increase its concentration and therefore proportionally increase the reaction rate.
It is commonly observed that an enzyme binds poor substrates which have low reaction rates, as well as or even better than good ones which have high reaction rates. thus, a good substrate doe not necessarily binds to its enzyme with high affinity, but it does so on activation to the transition state.





No comments:
Post a Comment